Product Range
The Galaxy Intelligent Cassette (iCassette)
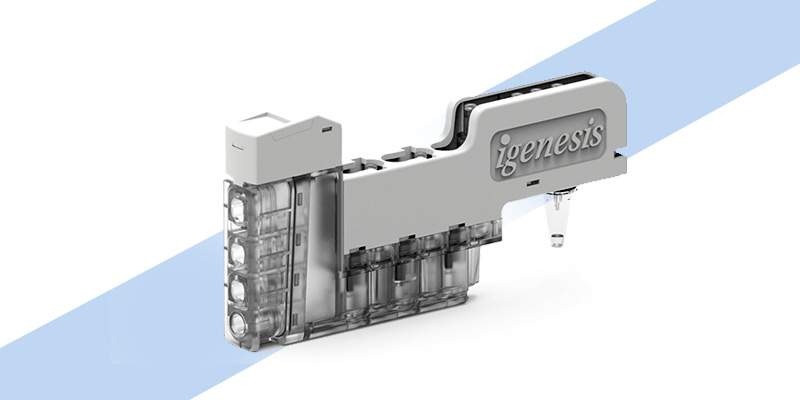
iCassette innovatively integrates all the experimental reagents, procedures, and lab environments required for nucleic acid extraction, purification, and PCR amplification into a small fully enclosed cassette.
More DetailsSARS-2-CoV (Covid-19) Detection - 1

SARS-2-CoV(Covid-19) nucleic acid detection cartridge (Real-time reverse transcription PCR method)
..
..
SARS-2-CoV (Covid-19) Detection - 2

SARS-2-CoV(Covid-19)/Influenza A Virus/Influenza B Virus/Respiratory Syncytial Virus Diagnostic Kit (PCR-Fluorescence)
..
Gynaecologic Oncology

Diagnostic Kit for detecting of 16 high-risk and genotyping of 16&18 human papillomavirus (HPV) (PCR fluorescence probe method)
More DetailsIntestinal Infectious Disease

Coxsackievirus A6/A10/A16/Enterovirus 71 RNA Diagnostic Kit(PCR-Fluorescence)
Respiratory Infectious Disease - 1

Diagnostic Kit for Mycobacterium Tuberculosis (MTB) complex DNA and Rifampicin resistance Mycobacterium tuberculos (PCR-Fluorescence)
More DetailsRespiratory Infectious Disease - 2

Streptococcus Pneumoniae/ Klebsiella Pneumoniae/ Legionella Pneumophila/ Haemophilus influenzae/ Bordetella Pertussis Diagnostic Kit (PCR-Fluorescence)
More DetailsSexual Infectious Disease

Chlamydia trachomatis/Niesseria gonorrhoeae /Ureaplasma urealyticum Diagnostic Kit (PCR-Fluorescence)
More Details
